JAM掲載論文検索
Japanese Acupuncture and Moxibustion (Online)
JAM 2005;Vol.1:36-42
Assessment of the Function of Peritoneal Macrophages in Rats treated with Long Term Direct Moxibustion
MATSUO Takako, KASAHARA Yuki, KURIBAYASHI Koichi
Division of Immunology and Pathology, Kansai College of Oriental Medicine
Abstract
We analyzed the functions of peritoneal macrophages after the treatment of direct moxibustion in rats. Fluorescent latex beads were injected into the abdominal cavity after the final moxibustion. Eighteen hours after the injection, peritoneal exdative cells (PECs) were collected from rats, stained with phycoerythrin (PE) labeled MHC class II antibody and analyzed by flowcytometer (FCM). The expressions of IL-1beta, TNF-alpha, and iNOS mRNA in the PECs were assessed by RT-PCR. The ratio of beads-ingested peritoneal macrophages to non-ingested macrophages was reduced in moxibustion-treated rats, but the number of macrophages that expressed the MHC class II molecules appeared to be increased in the moxibustion group. However, neither data set was statistically significant. The expression of IL-1beta mRNA was enhanced in the moxibustion group, but there were no differences in expressions of TNF-alpha and iNOS mRNA. Thus, we suggest that direct moxibustion may influence the function of peritoneal macrophages in rats.
Key words: Moxibustion, Macrophage, Cytokine, Flowcytometer
Introduction
Moxibustion is a treatment in which a certain size of moxa is burned on the skin. This thermal stimulation regulates the immune system in the body1). There have been many reports in which performing moxibustion in animals and humans induced immune modulation in the recipients (2-9), but the mechanisms have not been elucidated since now.
Macrophages serve as phagocytic cells that ingest foreign antigens entering the body or antigen-presenting cells that present the ingested foreign antigens with the class II MHC molecules (10,11). Macrophages also function as regulatory cells that secret free radicals, cytokines, and prostaglandins to induce an inflammatory response (10,11). Because macrophages play a crucial role in the immune system, we focused on the function of macrophages when direct moxibustion was performed in rats. Here we analyzed the ability of phagocytosis and the expressions of mRNA of IL-1beta, TNF-alpha and inducible nitric oxide synthase (iNOS) as indexes of the function of macrophages. We also measured the levels of expression of class II MHC molecules on the surface of macrophages to assess the competence of antigen-presentation.
Materials and Methods
Animals
Male Wistar rats (aged 8 weeks, 200-250g) were purchased from Japan SLC Inc. (Hamamatsu, Japan) and used at 2 to 5 months of age.
Moxibustion
Two external points, between the eleventh and twelfth thoracic vertebrae (B20) and between the second and third lumbar vertebrae (B23), were selected as acupoints. Before the animals were treated with moxibustion, they were anesthetized by pentobarbital sodium at a concentration of 2 mg/kg and their backs were shaved. Moxibustion to the acupoints was performed with three cones (0.5 mg moxa/cone) per point in a day. The stimulation was carried out two times in a week for 3 months.
Intraperitoneal Injection of Fluorescent Latex Beads and Collection of PECs
For assessment of the phagocytic activities of peritoneal exdative cells (PECs), fluorescent latex beads (2.6% solids, diameter 0.2 , Polyscience Inc., USA) were diluted with saline (final concentration 0.5%) and injected into the rat abdominal cavity12) after the final moxibustion. Eighteen hours after the injection, the PECs were collected by injecting 10ml of ice cold RPMI 1640 medium (Invitrogen Corp., Carlsbad, CA, USA) into the abdominal cavity.
Immunoflorescence staining and Analysis of PECs
Collected PECs were suspended in RPMI 1640 medium, passed through a nylon filter to remove debris, and washed with the medium. The cells were resuspended with medium at a concentration of 1,000,000 cells/ml. They were reacted with phycoerythrin (PE)-labeled anti-rat MHC class II monoclonal antibody (RT1B, OX-6, BD Biosciences Pharmigen, USA) for 30 minutes on ice, then washed 3 times with the medium. As a control, PECs that were not reacted with antibody were prepared. The uptake of fluorescent latex beads and the expression of MHC class II antigens in these PECs were analyzed by flowcytometer (Ortho Cytoron Absolute, Ortho-Clinical Diagnostics Inc., Tokyo, Japan). The ratio of the uptake of fluorescent latex beads in the macrophages was calculated the lower right (LR) / the lower left (LL) in the histogram of FCM. The ratio of MHC class II positive cells was calculated the upper right (UR) / the upper left (UL) in the histogram. The position of histogram was shown in figure 2.
Preparation of films of PECs
The films of PECs were made on glass slides and were stained with May-Grunwald-Giemsa stain.
Assessment of the Expression of Cytokine mRNAs
The expression of cytokine mRNA in PECs was assessed by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA from PECs was extracted by the acid guanidinium thiocyanate-phenol-chlorofolm method as follows13). A pellet of PECs was added to 500 of denaturing solution (4 M guanidine thiocyanate, 0.5% sarcosyl, 1M sodium citrate pH 7.0 and 0.1 M 2-mercaptoethanol), 50 of 2 M sodium acetate (pH 4.0), 500 of liquefied phenol, and 150 of chloroform. After incubation for 15 minutes on ice, the mixture was centrifuged at 10k rpm for 20 minutes and the aqueous phase was transferred to a fresh tube. Then 600 of isopropanol was added for salting out the RNA and the mixture was kept for 1 hour at -20 degrees centigrade. After centrifugation, the RNA pellet was obtained and washed with 80% ethanol. The pellet was dissolved in diethyl pyrocabonate-treated water, and the concentration of RNA of each sample was measured by absorbance at 260 nm (GeneQuantpro, Amersham Biosciences Corp., Piscataway, NJ, USA).
Reverse transcription was carried out under the following conditions14). Two micrograms of total RNA was mixed with 50 mM Tris-HCL (pH 8.3), 75 mM KCL, 3 mM MgCl2, 0.01 Mdiethiothretol, 10 U/ reverse transcriptase (SuperScriptII, invitrogen Corp., Carlsbad, CA, USA), 50 pmol/ random hexamers (Takara Shuzo Co., Ltd, Kyoto, Japan), and 2.5 mM/ dNTP (Amarsham Bioscienses Corp., Piscataway, NJ, USA). The mixture was kept for 1 hour at 37 to synthesize cDNA.
The PCR that followed was performed with a reaction mixture composed of synthesized cDNA, 2.5 mM/ dNTP, 10 mM Tris-HCL (pH 8.3), 50 mM KCL, 1.5 mM MgCl2, 25 pmol/ each primer and 10 U/ taq DNA polymerase (Applied Biosystems, Branchburg, NJ, USA) (15). The cDNA was amplified for 32 cycles with denaturation at 94 for 90s, annealing at 58 for 60s, and extension at 72 for 120s. The products of PCR were analyzed on a 3% agarose gel stained with ethidium bromide. The primers used are shown in Table 1.
Statistical analysis
Data were presented as mean } standard deviation (SD). The Studentfs t-test was used to determine the levels of significance of difference. Differences at p < 0.05 were considered statically significant.
Results
Phagocytosis of latex beads and expression of MHC class II molecules in PECs
The fluorescent latex beads were injected into the rat abdominal cavity to examine the phagocytotic ability of the peritoneal macrophages. Figure 1a shows the films of PECs collected from rats injected with fluorescent latex beads. There were many peritoneal macrophages with some lymphocytes and neutrophils. The macrophages have abundant cytoplasm with scattered small azurophilic granules. Their nuclei are eccentric in position and oval or reniform. Figure 1b is the film of the same place as figure 1a in the fluorescence microscope. Some of the macrophages ingested the latex beads in their cytoplasm.
PECs that ingested the fluorescent latex beads were easily detected by flowcytometry and 99.4 ~ 99.8% of gated PECs were CD11b positive (Figure 2). Flowcytometric analysis of PECs revealed that the number of cells in the macrophage fraction was decreased in the rats treated with moxibustion (p=0.025, Figure 3). There were no significant difference, but the ratios of bead-ingested macrophages to non-ingested macrophages were also reduced in the rats treated with moxibustion compared to those in non-treated rats (p=0.21, Figure 4a). The amount of latex beads ingested by peritoneal macrophages was assessed by measuring the mean channel of the fluorescence intensity of these macrophages. As shown in figure 4b, the amount of ingested beads in peritoneal macrophages was not different between the moxibustion and non-moxibustion groups (p=0.27).
For the assessment of the ability of antigen presentation in peritoneal macrophages, PECs were stained with PE-labeled anti-rat MHC class II monoclonal antibody. There were no significant difference in the ratios of MHC class II molecules-expressed peritoneal macrophages to non-expressed peritoneal macrophages because of the dispersion of the data (p=0.42, Figure 5a).
We also evaluated the numbers of MHC class II molecules expressed on the surface of the macrophages that ingested the latex beads by measuring the mean channel of FL-2 fluorescence intensity of these macrophages. However, no difference was found in the numbers of MHC class II molecules expressed between the moxibustion and non-moxibustion groups (Figure 5b).
Expression of Cytokine mRNA
For assessment of the expression of cytokine mRNA, total RNA extracted from PECs was amplified by RT-PCR. As shown in figure 6, the expression of IL-1beta mRNA in PECs from moxibustion-treated rats was enhanced compared to that in PECs from non-treated rats. There were no differences in the amounts of mRNA for TNF-alpha and iNOS between the moxibustion group and the non-moxibustion group.
Discussion
It is known that treatment with moxibustion improves immune functions of the body. Various studies into the effects of moxibustion on modulation of immune functions have been reported (2-9), but investigations of the macrophage function in the moxibustion-treated host are very rare (15).
Macrophages are phagocytic cells and serve to protect against infection in the early phase of immune response (10,11). Blood monocytes migrate into the connective tissue of the dermis or other organs and differentiate into macrophages. When foreign antigens enter into the body, the resident macrophages in the tissue ingest the pathogen by opsonization and are activated to secret factors with various biological activities which include the chemotaxis of neutrophils and lymphocytes, activation of endothelial cells of the vessels, and killing the bacteria with free radicals, enhancing the elimination of the pathogen. The macrophages also have the ability of antigen presentation. The macrophages degrade the ingested pathogen into peptide fragments and present these fragments with the class II MHC molecules to the helper T cells. This result in the activation of specific immunity called acquired immunity. Thus the macrophages are major inductive and effector cells in the immune system, and play a crucial role in the protective function of the body. We thus decided to investigate the macrophage functions when the hosts were treated with moxibustion.
Because it is rather difficult to handle macrophages in in vitro experiments compared to other leukocytes, there are few assays that observe the function of macrophages. Steinkamp et al. reported that the ability of phagocytosis of macrophages could be assessed by measuring the fluorescence intensity of alveolar macrophages that ingested fluorescent latex beads using flowcytometric analysis12). So, according to the methods of Steinkamp et al., we carried out an assay of phagocytotic ability of the intraperitoneal macrophages that ingested fluorescent latex beads (13).
In the present experiments, however, the phagocytic activities of macrophages were not enhanced by moxibustion treatment when we assessed the ratio of bead-ingested cells to non-ingested cells among the PECs and the amount of ingested beads in each macrophage. Furthermore, the numbers of peritoneal macrophages recovered from peritoneal cavities were rather decreased in the moxibustion group compared to those in the non-moxibustion group. Tohya et al. reported that macrophages accumulated in the dermis when the moxibustion was performed for a long period of time (8). They suggested that these accumulations of macrophages were due to the elevated expression of intracellular adhesion molecule-1 (ICAM-1) on peculiar vessels in the dermis that were similar to high endothelial venules in lymph nodes. Thus, the decreased numbers of PECs recovered from peritoneal cavity might have been due to the migration of many macrophages to stimulated sites in the skin.
The macrophages that ingested beads showed a tendency of increased expression of class II MHC molecules although the differences between moxibustion-treated and non-treated groups were not statistically significant. These results might indicate that the moxibustion treatment enhances the antigen-presentation ability of macrophages when they ingest the antigens.
PECs recovered from the rats treated with moxibustion showed increased expression of IL-1beta mRNA. IL-1 induces chemotaxis of macrophages and neutrophils, and enhances expression of ICAMs in endothelial cells, resulting in induced inflammation and enhanced protection against microbial invasion. Thus, the increased expression of IL-1beta mRNA in PECs might have enhanced the protective activity of macrophages in the rats treated with moxibustion, but it might have been inflammatory reaction by thermal burn of moxibustion (19). However, the long term direct moxibustion that we performed was considered validly, because Kanai et al. (17) and Matsukuma et al. (18) had been reported that the moxibustion for a long period of time was improved arthritis of rat.
Although the present data may suggest the enhancement of immunity by the direct moxibustion treatment, there were some rats that showed intermittent diarrhea and became to have a bad fur among moxibustion-treated rats. Chronic stimulation (three months) with direct moxibustion may be too strong for rats that are about much smaller (about 1/250) than humans in weight, even if the size of moxibustion could be diminished in proportion to the body size of the rats. We will in future investigate the effects of indirect moxibustion, as frequently performed in humans, on the immune functions in rats.
References
1) Amagai T, Itoi M. The immune system and acupuncture moxibustion. J Jpn Soc Acupunct Mox. 1996; 46(4): 315-25. Japanese.
2) Hau D-M, Wu J-C, Chang Y-H, Hwang J-T. Effects of moxibustion on cellular immunocompetence of -irradiated mice. Am J Chin Med. 1989; 17: 157-63.
3) Utsunomiya Y. Effect of moxibustion on the growth of primary tumor, pulmonary metastases and immune responses in C57BL/6 mice bearing Lewis lung carcinoma (LLC). Bull Meiji Univ Orient Med. 1995; 16: 27-38. Japanese.
4) Yamashita H, Takahashi M, Nishijo K, Ichiman Y. Effect of moxibustion on serum antibody production in rabbits immunized with Staphylococcus aureus. J J Orient Med. 1996; 47: 457-64. Japanese.
5) Chiba A, Nakanishi H, Chichibu S. Effect of indirect moxibustion on mouse skin. Am J Chin Med. 1997; 25: 143-51.
6) Chiba A, Nakanishi H and Chichibu S. Thermal and antiradical properties of indirect moxibustion. Am J Chin Med. 1997; 25: 281-7.
7) Yamashita H, Tanno Y, Ichiman Y, Nishijo K, Takahashi M. Influence of direct moxibustion with moxa cones the size of a grice grainh on cell count and proportion of leukocytes in rabbit and human peripheral blood. J J Orient Med. 1998; 48: 599-608. Japanese.
8) Tohya K, Urabe S, Igarashi J, Tomura T, Take A, Kimura M. Appearance of peculiar vessels with immunohistological features of high endothelial venules in the dermis of moxibustion-stimulated rat skin. Am J Chin Med 2000; 28: 425-33.
9) Kasahara Y, Matsuo T, Kuribayashi K, Kawamoto M, Tohya K, Kimura M. Effects of indirect moxibustion on the expression of cytokine mRNAs in human lymphocytes. Annu Rep Kansai Coll Orient Med. 2001; 17: 42-50. Japanese.
10) Gordon S. Macrophages and the immune response. In: Paul WE (ed) Fundamental Immunology. 5th ed. Philadelphia. Lippincott Williams & Wilkins. 2003: 481-95.
11) Goldsby RA, Kindt TJ, Osborne BA. Mononuclear cells. In: Cells and organs of the immune system. Kuby Immunology. 4th ed. New York. W. H. Freeman and Company. 2000: 41-4.
12) Steinkamp AJ, Wilson SJ, Saunders CG, Stewart CC. Phagocytosis: Flow cytometric Quantitation with fluorescent microspheres. Science. 1982; 215: 64-6.
13) Matsuo T, Kasahara Y and Kuribayashi K. Assessment of natural immunity by the analysis of functions of activated peritoneal macrophages. Bull Kansai Coll Orient Med. 2004; 1: 25-30. Japanese.
14) Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-chloroform extraction. Anal Biochem. 1987; 162: 156-9.
15) Kawasaki ES. Amplification of RNA. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds). PCR protocols. A guide to methods and applications. Calfornia. Academic Press, Inc. 1990: 21-7.
16) Okazaki M, Furuya E, Kasahara T, Sakamoto K. Effects of single moxibustion on phagocytic activity in mice. Am J Chin Med. 1983; 11: 112-22.
17) Kanai S, Take A, Taniguchi N. Study of moxibustion for experimental arthritis model rat. Orient Med Pain Clin. 2000; 30: 16-21. Japanese.
18) Matsukuma H, Shinohara K and Nakamura T. Preventive effect of moxibustion on collagen-induced arthritis in mice. Bull Meiji Univ Orient Med. 2002; 30: 9-19. Japanese.
19) Dinarello CA. Interleukin-1 family of ligands and receptors. In: Paul WE (ed). Fundamental Immunology. 5th ed. Philadelphia. Lippincott Williams & Wilkins. 2003: 775-82.
Figure legend
Fig. 1. Phagocytosis of peritoneal macrophages
a. PECs were stained with May-Grunwald-Giemsa stain. X100 b. PECs of the same place as 1a were seen in fluorescence microscope. This film shows that some of peritoneal macrophages ingested the latex beads in their cytoplasm. X100
Fig.2. FCM image of CD11b positive cells. This image is the fluorescent latex beads ingested PECs that stained PE labeled CD11b antibody. The most of PECs were shown CD11b positive and 99.4-99.8% of them involved in upper right of histogram.
Fig.3. The number of cells (a) and percentage of positive cells (b) in gated area were analyzed by flowcytometry. The results are the means of 4-5 rats per group. Bars show + SD.
Fig.4. Ability of phagocytosis of peritoneal macrophages after direct moxibustion for 3 months. The rats were injected with latex beads into the abdominal cavity after final moxibustion. PECs collected from rats were analyzed by flowcytometry. a. The ratios of bead-ingested macrophages to non-ingested macrophages. The results are the means of 4-5 rats per group. Bars show + SD. b. The mean channel of the fluorescence intensity of these macrophages. The results are the means of 4-5 rats per group. Bars show + SD. N. S. : not significant
Fig.5. Expression of MHC class II molecules on the surface of peritoneal macrophages after direct moxibustion for 3 months. PECs were collected from the rats injected with latex beads into the abdominal cavity after final moxibustion and stained with PE-labeled anti-rat MHC class II monoclonal antibody. a. The ratios of MHC class II-expressed macrophages to non-expressed macrophages in bead-ingested macrophages. The results are the means of 4-5 rats per group. Bars show + SD. b. Assessment of the mean channel of the fluorescence intensity of these macrophages is shown as the mean in each group. The results are the means of 4-5 rats per group. Bars show + SD. N. S. : not significant
Fig.6. Assessment of cytokine and iNOS mRNA in PECs. PECs were collected from the rats injected with latex beads into the abdominal cavity after final moxibustion. Total RNA was extracted from these cells and the expression of cytokine and iNOS mRNA was assessed by RT-PCR (lanes 1-5, non-moxibustion group; lanes 6-10, moxibustion group).
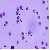
Fig. 1a. Phagocytosis of peritoneal macrophages

Fig. 1b. Phagocytosis of peritoneal macrophages

Fig.2. FCM image of CD11b positive cells.
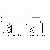
Fig.3. The number of cells (a) and percentage of positive cells (b) in gated area were analyzed by flowcytometry.

Fig.4. Ability of phagocytosis of peritoneal macrophages after direct moxibustion for 3 months.
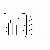
Fig.5. Expression of MHC class II molecules on the surface of peritoneal macrophages after direct moxibustion for 3 months.

Fig.6. Assessment of cytokine and iNOS mRNA in PECs. PECs were collected from the rats injected with latex beads into the abdominal cavity after final moxibustion.